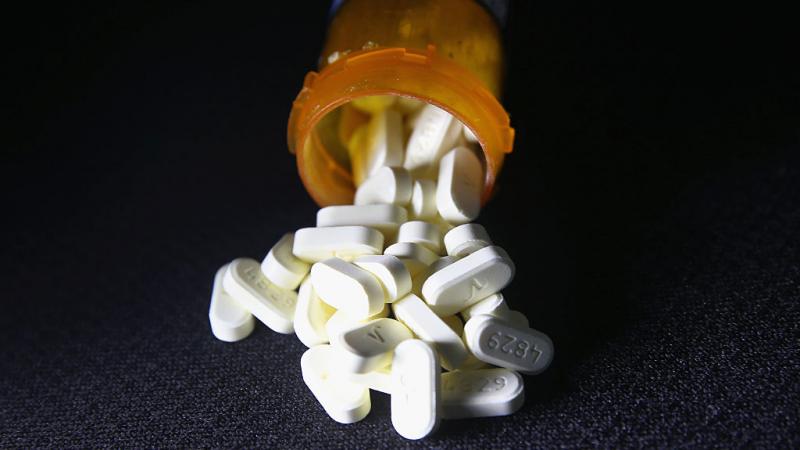
Eight boosted mice: Critics pan FDA for preelection Omicron jab approval based on irrelevant data
Epidemiologist says using BA.1 data to approve BA.4/5 booster is like equating pizza and cake. COVID subcommittee chair cheers approval on eve of midterms after denouncing Trump for pressure to approve vaccines before 2020 election.
Mainstream media have joined prominent doctors in questioning the FDA for its reliance on animal studies to approve so-called bivalent COVID-19 boosters for ages 12 and up, even as the shots are already being forced on low-risk, "fully vaccinated" populations such as college students.
Commissioner Robert Califf and Peter Marks, director of the Center for Biologics Evaluation and Research, said the FDA was using the same process for boosters combining ancestral and currently dominant Omicron BA.4/5 components that it follows for annual flu shots.
"This is the number one question people are asking," Califf said in a press conference Wednesday. The agency has "extensive experience in the past with strain changes made without clinical data based on the totality of available evidence," Marks said, citing flu vaccines.
The FDA didn't give Just the News access to the interactive Zoom feed to ask questions, just the one-way YouTube stream, and didn't answer requests for the evidence behind their claims.
Wake Forest University imposed the bivalent boosters nearly a month before their approval "to strengthen our community's collective immunity," with no exemption for even recently boosted community members. It hasn't yet decided whether to renew its flu vaccine mandate.
The Mercury News emphasized that bivalent booster "studies about efficacy, so far, have only been completed in mice." Eight mice in Pfizer's study "generated a 2.6-fold increase in neutralizing antibody levels" against BA.4/5 compared to its current booster, while "lab work" showed Moderna's caused an 8-fold increase.
Those figures were left out of the FDA's press release on its emergency use authorization of the two boosters, as was any mention that the "nonclinical data" came from animals.
CDC Director Rochelle Walensky said the feds didn't have time to wait on human data. Otherwise, "we will be using what I would consider to be a potentially outdated vaccine" for an expected COVID surge, she told Conversations on Health Care.
This shows midterm elections are behind the rush to approve boosters, University of California San Francisco epidemiologist Vinay Prasad tweeted. Unless agencies are "firewalled" from politics, the feds will "approve anything to lower cases right before the vote" regardless of safety concerns.
Select Subcommittee on the Coronavirus Crisis Chair Jim Clyburn (D-S.C.), who successfully pressured the FDA to lower its standards for COVID vaccines for children under 5 this spring, issued a statement Wednesday celebrating the approval of bivalent boosters.
A week earlier, the subcommittee denounced the Trump administration for pressuring regulators to approve COVID vaccines ahead of the 2020 election. Spokesperson Graeme Crews didn't respond when asked how booster approval without human data on the eve of midterms was different.
Califf and Marks invoked their sister agency's new report on the second consecutive year of reduced life expectancy to justify bivalent booster approval.
"COVID was the main factor" in life expectancy falling from 77 years in 2020 to 76.1 years in 2021, according to Robert Anderson, chief of mortality statistics at CDC's National Center for Health Statistics, HealthDay reported. Other factors, including drug overdose and suicide, were "probably related to the pandemic, but not directly to the virus."
It's not clear bivalent boosters would reverse or mitigate that trend, however. The steepest drop in life expectancy was in the category "American Indian and Alaska Native," which fell 1.9 years to 65.2 in 2021.
AIAN has a higher booster rate than the black and Hispanic/Latino categories, according to the latest CDC data from Aug. 24. Black life expectancy declined 0.7 years to 70.8, and Hispanic/Latino 0.2 years to 77.6. Whites, whose booster rate is second only to the Asian category, lost a full year, to 76.4.
The FDA officials repeatedly invoked the prospect of rising hospitalizations and deaths without bivalent approval in the press conference. "We're not talking about trivial things," Califf said. Marks said he was hearing from doctors that pediatric hospitals are admitting more children while adult hospitalizations fall.
But the FDA isn't explicitly claiming the new boosters actually reduce severe outcomes. It cited clinical studies of each vaccine maker's Omicron BA.1 bivalent, which found "better ... immune response" from the bivalent than the single-strain booster. (Moderna's version was approved by the U.K. last month.)
While the agency claims BA.1 evidence is enough to approve BA.4/5 boosters "because these vaccines are manufactured using the same process," Prasad said this was like equating pizza and cake because they are made "in the same oven."
The agency's reliance on antibody response isn't echoed by Pfizer, whose vaccine R&D chief told the agency's outside advisers last year that it hadn't found an "antibody threshold" for breakthrough infections. Kathrin Jansen said there's "probably a much more complex story and not easily just addressed with neutralizing antibodies."
The agency shut out the Vaccines and Related Biological Products Advisory Committee this time, with Califf explaining that VRBPAC approved bivalent boosters in theory in June. The Mercury News reported that VRBPAC member Paul Offit of Children's Hospital of Philadelphia disapproved of mouse-only data. He didn't answer Just the News queries.
"Americans deserve to see the data," Johns Hopkins medical professor and National Academy of Science member Marty Makary tweeted, asking why the White House and COVID adviser Ashish Jha are "pushing [bivalent boosters] so hard & universally w/o public data?"
"How did we go from 40k person [randomized controlled trials] to 8 mice," which is too small to determine whether the new boosters reduce or worsen the heart inflammation side effect from current vaccines, Prasad tweeted.
Califf and Marks said the risk of myocarditis prompted them to choose a two-month window between doses. Young people, who are at highest risk of the side effect, shouldn't wait longer because they need to "refresh the immune system with what's actually circulating," and the booster can "probably reduce by more than half their risk of long COVID," Marks said.
The Facts Inside Our Reporter's Notebook
Videos
Links
- questioning the FDA for its reliance on animal studies
- long deemed misinformation
- nearly a month before their approval
- The Mercury News
- FDA's press release
- Conversations on Health Care
- Vinay Prasad tweeted
- pressured the FDA to lower its standards
- subcommittee denounced the Trump administration
- HealthDay reported
- latest CDC data from Aug. 24
- approved by the U.K. last month
- Prasad said this was like equating pizza and cake
- vaccine R&D chief told the agency's outside advisors last year
- Califf explaining
- Marty Makary tweeted
- Prasad tweeted